When the average cost of a data breach in the pharmaceutical industry is $4.82 million, it's clear that redaction software is the need of the hour.
The pharmaceutical sector faces a critical challenge in safeguarding valuable information, as there is much of it in current and archived files.
From private information of patients and test subjects to confidential company files, these secrets are ripe for the picking of any malicious actor.
But it's not just the information that gets stolen. When sharing reports or files with regulators and the public, companies accidentally reveal sensitive information like PII, PHI, NPI, PCI, and CCI. This poses an extra risk in an industry where keeping things private is crucial.
This blog will explore what kind of information pharmaceutical companies should keep confidential. We'll also discuss how to ensure it with the power of redaction software.
What is Redaction Software?
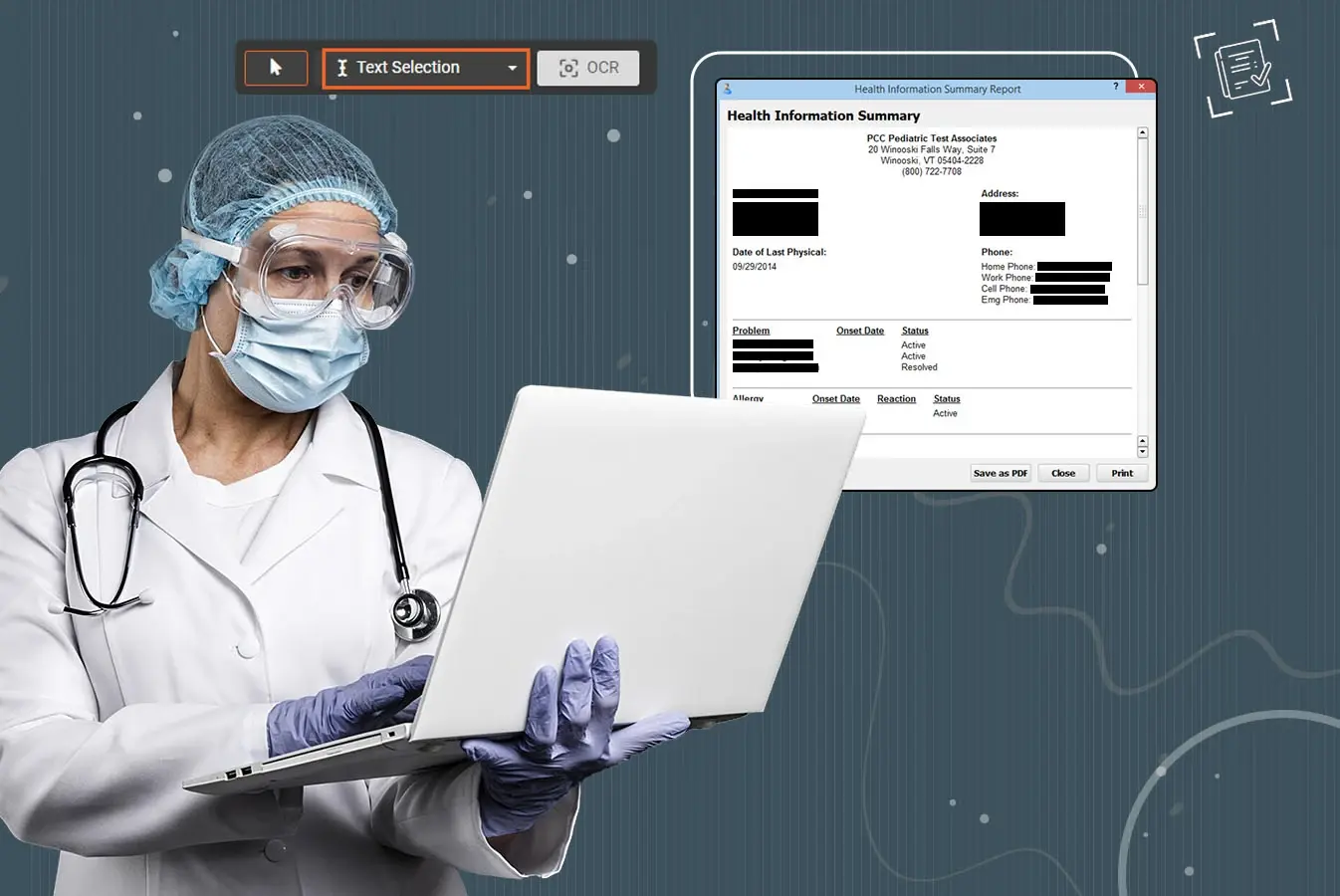
Redaction software is designed to selectively remove or obscure sensitive information from documents, images, audio, and videos.
This process protects confidential data before sharing documents or files with other stakeholders.
The Sensitive Data that Pharmaceutical Companies Carry
Pharmaceutical companies often must share clinical trial data with regulatory authorities before making it public. This ensures that the drug research is transparent and available for review.
Before a drug can be approved, marketed, and sold, it must undergo a rigorous evaluation process. This involves a detailed assessment of preclinical and clinical data.
The goal is to assess whether the proposed medicine meets the necessary quality, safety, and effectiveness standards.
If you're an American pharma company, you must submit your data to the Food and Drug Administration (FDA).
But if you're in the EU, you submit it to the European Medicines Agency (EMA) and individual member states' National Competent Authorities (NCAs).
While this all sounds good on paper, there's a problem. These reports inadvertently end up containing Commercially Confidential Information (CCI).
CCI is any information that could reveal a company's trade secrets, proprietary processes, or invaluable research data. In essence, it's the kind of information that details a company's roadmap to success.
What is Redaction in Clinical Trials?
Now that we've established that clinical trial documents contain Commercially Confidential Information (CCI), the question is, why redact them?
The biggest reason is to stop a competing pharmaceutical company from accessing these publicly disclosed trials and extracting confidential or proprietary information.
Think about it. If a pharmaceutical company finds an edge over its competitors, what stops it from taking advantage? Well, not much.
Anything that gives away company secrets, processes, or valuable research data can fall at risk of being disclosed publicly. And if it falls into the hands of a competitor, it can spell severe consequences.
This is why pharmaceutical companies must go through their reports with a fine-toothed comb. Their only option is to redact Commercially Confidential Information (CCI) in clinical reports before submission.
What is Commercially Confidential Information (CCI) in Clinical Reports?
Commercially Confidential Information (CCI) contains information about the specific process, formulation, or techniques involved in the production of drugs.
As you might expect, manufacturing drugs isn't easy. It involves careful consideration of active ingredients, how they should be formulated, and any advanced techniques used in making them.
While Commercially Confidential Information (CCI) is broad, some common types can be found in clinical reports.
The first involves the specific combination of ingredients, their proportions, and the manufacturing processes that give a particular medicine unique characteristics.
The second includes techniques and procedures used to produce pharmaceuticals. These often involve intricate processes contributing to a drug's quality and efficacy.
Of course, these aren't the only types of Confidential Information (CCI) — It includes anything that qualifies as groundbreaking insights, novel approaches, sponsor data, or undisclosed findings.
For more information on specific Commercially Confidential Information (CCI) redaction, refer to the European Medicines Agency's (EMA) handbook.
What Doesn't Count as Commercially Confidential Information (CCI) in Clinical Reports?
According to the European Medicines Agency (EMA), certain information does not qualify as Commercially Confidential Information (CCI). This includes:
Information that counts as Public Interest
- General or administrative details such as names of vendors and Contract Research Organizations (CROs)
- Information related to the quality of drug formulations, such as duration of storage, humidity, temperature, and more
- Non-clinical information such as pharmacological tests and methods, quantification range, etc.
- Clinical information such as protocols or statistical methods for data
Information available within the Public Domain
- Scientific literature and articles (such as Textbooks, PubMed, and Medline)
- Scientific guidelines
- Company website
- Patent application
- Clinical trial registries
- Websites of other regulatory authorities within and outside of the EU.
Information that counts as Public Knowledge
- Treatment guidelines
- Scientific literature and articles (Textbooks, PubMed, Medline)
- Scientific and regulatory guidelines
Redaction for HIPAA Compliance
Data redaction isn't done just to protect trade secrets and company information. Sometimes, there are other factors to consider, such as privacy concerns.
Pharmaceutical companies and healthcare organizations must redact Protected Health Information (PHI) and Personally Identifiable Information (PII) from media files.
Usually, this sensitive information is found in medical records, clinical notes, prescriptions, health insurance documents, and more.
However, pharmaceutical companies may find this information in clinical trial documents, employee records, health records, and more.
To ensure the privacy of patient information, Pharmaceutical companies must redact this information to comply with the Health Insurance Portability and Accountability Act (HIPAA).
If they don't? They risk severe non-compliance penalties, legal action, and a loss of reputation.
How Pharmaceutical Companies Can Redact Data Effectively Using Redaction Software
Pharmaceutical companies face significant challenges when redacting medical records and clinical trial documents.
For instance, manually redacting many documents and files can be a challenge for pharmaceutical companies.
By the same token, manual redaction can often leave room for error. When this happens, it can increase the risk of missing or overlooking sensitive data. This can spell disaster when the goal is to meet stringent compliance.
To save time, effort, and money, pharmaceutical companies often look to redaction software to pick up the slack. This allows them to easily redact data quickly and without any errors.
Armed with the best redaction software, any pharmaceutical company can streamline its redaction process.
Redact Confidential Information with VIDIZMO Redactor
Don't let the weight of redaction burden your organization and its clinical trial disclosure specialists. VIDIZMO Redactor is your solution for quick, easy, and seamless compliance and data redaction.
With VIDIZMO Redactor, all it takes is a few clicks to redact data from images, videos, documents, and audio. It's the all-in-one redaction software your organization needs to safeguard its secrets and stay compliant.
Here's how VIDIZMO Redactor can help your organization:
- Redact in Bulk: Never deal with redacting your files one by one ever again. Simply select multiple files at once and redact them in one go.
- Redact Scanned Documents with Optical Character Recognition (OCR): Dealing with thousands of images of scanned documents? No worries. Detect text within them with AI and redact with ease.
- Define Your Own Redaction Rules: Create your custom criteria for what to redact using regular expressions. Then, with just a few clicks, you can instantly remove it from your files.
- Take Control with Manual Redaction: Use intuitive manual redaction tools to target your redaction in specific areas.
- Generate a Redacted Copy: Generate a redacted copy after redacting while keeping the original intact.
- Search Files with AI-Enhanced Search: Search through extensive file records using tags, spoken words, metadata, and more.
Take control of your data protection strategy with VIDIZMO Redactor. Empower your clinical trial disclosure specialists to handle redaction with confidence. Get started with our 7-day free trial today.
Still need to decide. Talk to us today to see what VIDIZMO Redactor can do for you.
Protect Your CCI Now
Pharmaceutical companies have a lot to grapple with. There's a major duty to care for the public, keep secrets safe, and follow strict regulations. It's not always easy to achieve a balance between the three.
Luckily, redaction software is the solution that helps achieve all these goals. Tools like VIDIZMO Redactor help pharmaceutical organizations meet their needs for precise data protection and regulatory compliance.
FAQs
What should be redacted from medical records?
Sensitive information such as patient names, addresses, and other identifying details should be redacted from medical records. Even the slightest of instances left behind may result in hefty penalties and beyond-repairable reputation damage.
What is the purpose of redacting a document?
Redacting documents aims to protect confidential information and comply with privacy regulations by obscuring sensitive details.
How does redaction software work?
Redaction software uses Artificial Intelligence (AI) to identify and mask specific information, ensuring accurate and efficient removal of sensitive data. Using redaction software also lets you take control of the redaction process without resorting to redaction services from third parties.
Why are records redacted?
Records are often redacted to prevent unauthorized access, protect privacy, and comply with legal and regulatory requirements.
What information needs to be redacted?
Information that needs to be redacted includes sensitive data like personal details, financial information, or proprietary content that should not be disclosed.
What are the principles of redaction?
The fundamental principles of redaction include removing or masking sensitive information while maintaining the integrity of data and protecting the privacy of others.
Posted by Rafay Muneer
Rafay is a Senior Product Marketing Strategist at VIDIZMO. He is driven to explore data protection and redaction solutions across various sectors. For any inquiries or assistance, feel free to get in touch at websales@vidizmo.com.